Abstract
BackgroundTP53-mutated (m) acute myeloid leukemia (AML) is a high-risk disease with sub-optimal response to conventional therapies and portends a particularly worse outcome in patients with relapsed or refractory disease. In this study we analyzed the outcomes of patients with TP53m AML after first relapse or after primary induction failure.
Methods We conducted this retrospective study through COMMAND (Consortium on Myeloid Malignancies and Neoplastic Diseases), a collaboration involving 10 US academic institutions. 366 patients with TP53m AML diagnosed between 2012-2021 were evaluated with 151 patients who had either primary refractory (87 [58%]) or relapsed (64 [42%]) disease and received further care, were the subject of this study. Continuous variables were summarized as median (range), while categorical variables were reported as frequency (percentage). Predictors of treatment response were assessed by Chi-square or Fisher's exact test for nominal data and Wilcoxon rank-sum test for continuous variables. Event free survival (EFS) was estimated from time of diagnosis to progression or death. Overall survival (OS) from the date of first salvage until last follow up or death was evaluated by the Kaplan-Meier method with differences compared by the log-rank test. Cox proportional hazards regression models were used to find the univariate and multivariate predictors of OS. Multivariable models included all significant univariate predictors with p value <0.1.
Results Baseline characteristics
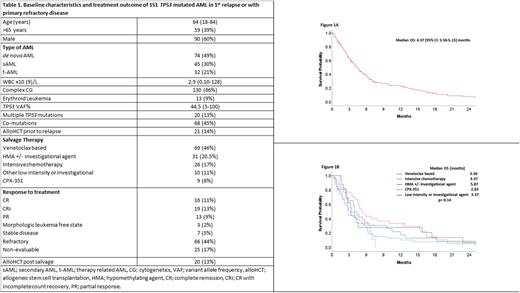
Baseline characteristics and treatment outcome are summarized in Table 1. The median age of the patients was 64 (range [R], 18-84) years and 59 (39%) patients were > 65 years of age. 21% of patients had therapy-related AML, 86% had complex cytogenetics (CG) and 9% of these patients had erythroid leukemia. Twenty-one (14%) patients relapsed after allogeneic stem cell transplantation (alloHCT). Co-mutations were observed in 45% of patients with the most frequently occurring (≥ 5% of patients) being ASXL1 (5%), TET2 (7%), DNMT3A (8%), RUNX1 (7%), RAS (7%), and JAK2 (8%) mutations. Salvage therapies were venetoclax-based (with hypomethylating agent [HMA] or low dose cytarabine [LDAC]) (46%), HMA +/- investigational agent (20.5%), intensive chemotherapy (IC; excluding CPX-351) (17%), other low intensity +/- investigational agent (11%) and CPX-351 (6%).
Responses
The rate of complete remission (CR) with or without count recovery (CRi) was 24% (n= 35) and 13% (n=20) were able to receive alloHCT after achieving response. We evaluated predictors of response (CR/CRi) using baseline variables. Age > 65 yrs (23% vs 77%, p=0.03) and secondary AML (15% vs 85%, p= 0.02) were significantly associated with an inferior response rate. Remaining variables were not significantly associated with response to salvage therapy.
Survival
The median EFS was 3.97 months (95% CI: 31.5-4.78) and median OS was 4.37 months (95% confidence interval [CI]: 3.58-5.15) (Figure 1a). In sub-group analysis, median OS (in months) based on salvage therapy was 3.23, 5.20, 3.93, 3.37 and 2.83 with venetoclax based, HMA +/- investigational agent, IC, other low intensity +/- investigational agent and CPX-351, respectively (p= 0.16) (Figure 1b). We did multivariate analysis (MVA) for OS, utilizing univariate predictors with p value <0.1. Achievement of CR/CRi (hazard ratio [HR]=0.57, 95% CI: 0.36-0.91; p=0.01) and alloHCT after salvage therapy (HR=0.45, 95% CI: 0.24-0.75; p= 0.03) retained significance for better OS. Complex CG (HR=1.83, 95% CI: 1.05-3.17; p= 0.03) and diagnosis of erythroid leukemia (HR=2.25, 95% CI: 1.22-4.17; p= 0.009) retained significance for inferior OS in MVA.
Conclusion As expected, our study confirms the dismal outcome of patients with TP53m AML progressing on first line therapy, however alloHCT can significantly improve outcome even after salvage therapy in a subset of patients who achieve response and are eligible for alloHCT. Effective therapies are urgently needed to improve outcome of patients with TP53m AML.
Disclosures
Atallah:Abbvie: Consultancy, Research Funding, Speakers Bureau; Takeda: Research Funding; Blueprint: Speakers Bureau; BMS: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding. Shallis:Bristol Myers Squibb and Gilead Sciences, Inc: Honoraria; Gilead Sciences, Inc.: Honoraria. Stahl:Novartis: Other: Advisory Board; Boston Consulting: Consultancy; Curis Oncology: Consultancy. Patel:Celgene/BMS: Research Funding; Pfizer: Research Funding; Servier/Agios: Research Funding; Kronos Bio: Research Funding; AbbVie: Consultancy. Abaza:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; ALX Oncology: Research Funding; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Duvall:Jazz Pharmaceuticals: Consultancy. Palmisiano:Genentech: Research Funding; AbbVie: Research Funding. Kota:Novartis: Honoraria; Pfizer Inc: Honoraria, Research Funding; Ariad: Honoraria; Incyte: Honoraria; Xcenda: Honoraria. Goldberg:Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Moderna, Novavax: Current equity holder in publicly-traded company; DAVA Oncology: Honoraria; Aprea, Aptose, AROG, Celularity, Pfizer, Prelude Therapeutics: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Litzow:Abbvie: Research Funding; Amgen: Research Funding; Astellas: Research Funding; Novartis: Research Funding; Syndax: Research Funding; Jazz: Consultancy; Actinium: Research Funding; Pluristem: Research Funding; Biosight: Other: Data Monitoring Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal